Biological macromolecules are organic meaning they contain carbon. All of these elements are nonmetals.
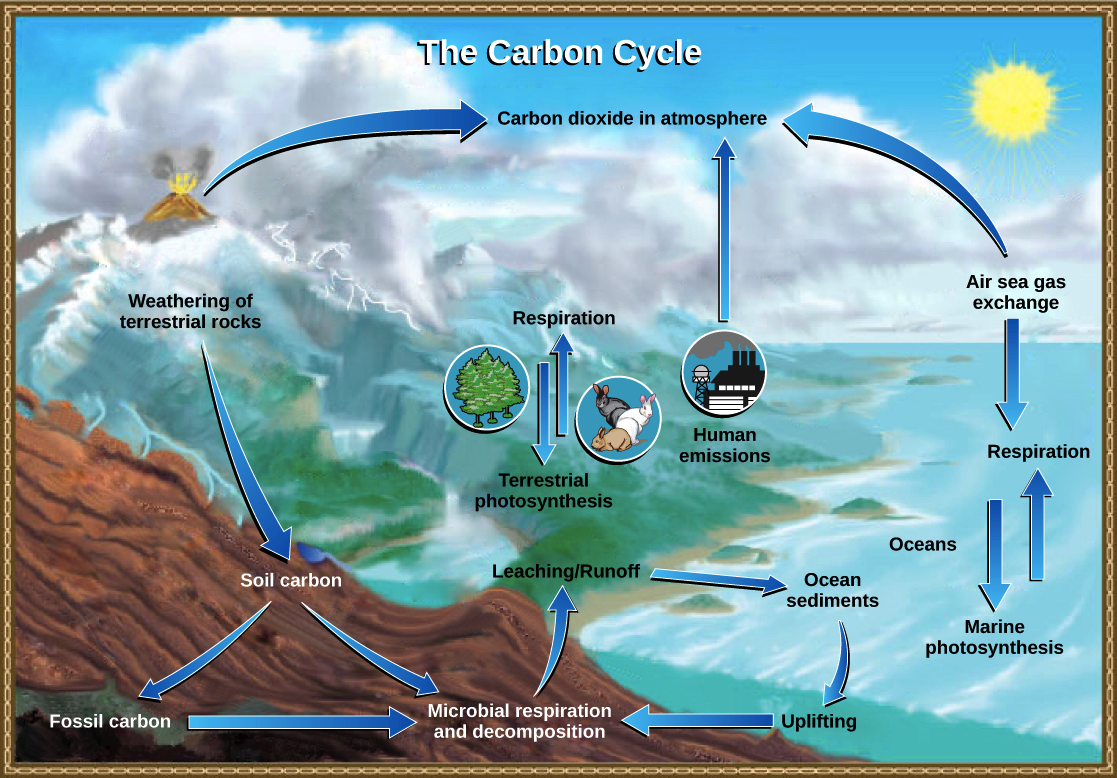
The Carbon Cycle Article Ecology Khan Academy
Eye color male or female fingerprint pattern tongue roll earlobe shape dimples and many other traits.
. Carbohydrates macromolecule Carbo means charcoal so think burnfuel. Carbon bonds are stronger than any other bond. The six neutrons in its nucleus the two electrons in its inner shell d.
N the H3CNCO molecule the carbon nitrogen and oxygen atoms are bonded in the order CNCO. The simplest carbon molecule is methane CH 4 depicted here. Carbon is a vital part of the phospholipids that make up cell membranes and the carbohydrates that make up cell walls.
- carbon has 4 valence electrons. The six protons in its nucleus b. The three elements that make up over 99 percent of organic molecules are carbon hydrogen and oxygen.
The feature of carbon important for producing a diversity of organic molecules is carbon can form both polar and nonpolar covalent bonds with various elements. Carbon can form hydrogen bonds with water. We group these organic compounds into four types.
Carbohydrates proteins lipids and nucleic acids. Organize the types of bonds according to the degree to which electrons are shared between two atoms. Which of the following statements best sums up the number of.
Furthermore what 3 elements do all organic molecules contain. They may be amorphous or even insoluble in water. They can be classified as monosaccharides disaccharides and polysaccharides.
The nucleic acids include DNA and RNA that are the polymers of nucleotides. Carbon can form up to two covalent bonds with other elements. The four electrons in its.
This gives cells the information they need to make protein and other parts of the cell. The Earth formed about 46 billion years ago. Carbohydrates and lipids are made of only carbon hydrogen and oxygen CHO.
The foremost common macromolecules in biochemistry are biopolymers and enormous non-polymeric molecules synthetic fibers furthermore as experimental materials like carbon nanotubes. Carbon is the main element of organic compounds we need to live. Such pathways include those involving nitric oxideguanylate cyclase reac.
Which of the following allows carbon to bond with up to four other atoms at a time and form the macromolecules that are essential to life. Carbohydrates monomer is Glucose which makes energy in our body. In this regard which macromolecule does not contain nitrogen.
Still wondering why is carbon so important in biology. Organic molecules are usually composed of carbon atoms in rings or long chains to which are attached other atoms of such elements as hydrogen oxygen and nitrogen. Carbohydrates are polymers of carbon hydrogen and oxygen.
This means that carbon atoms bonded to other carbon atoms or other elements form the fundamental components of many if not most of the molecules found uniquely in living things. There are four classes of macromolecules polysaccharides or carbohydrates triglycerides or lipids polypeptides or proteins and nucleic acids such as DNA RNA. What does this mean.
Name four groups of organic compounds found in living things. Carbon builds large molecules that are important for us. Describe at least one function of each group of organic compound.
Nucleic acid is a crucial class of macromolecules found in all told cells and viruses. The acronym CHNOPS which stands for carbon hydrogen nitrogen oxygen phosphorus sulfur represents the six most important chemical elements whose covalent combinations make up most biological molecules on Earth. It is because of the special binding properties of carbon that we get proteins carbohydrates lipids and nucleic acids each of which is an essential class of macromolecules to almost all living.
Why is glucose a macromolecule. In the following reaction compound Q is obtained from compound P via an ionic intermediate. They form many shapes like Branches rings and long chains.
Fatty acids and carbon are not macromolecules. Combined these molecules make up the majority of a cells dry mass recall that water makes up the majority of its complete mass. Carbon Makes Organic Molecules Why Carbon Carbon is the second most abundant element in living organisms Carbon can share four electrons therefore it can bond to four additional atoms Carbon establishes covalent bonds stable high energy bonds.
In the following reaction A is. In addition they may contain hydrogen oxygen nitrogen and additional minor elements. In the following questions two statements are given one is Assertion A and the other is Reason R.
Endogenous carbon monoxide CO is generated through the heme oxygenase-catalysed degradation of heme and is now established as an important biologically active molecule capable of modulating a number of signalling pathways. Draw the most important Lewis structure for the H3CNCO molecule and then answer the following questions. For about 500 million years the Earth was continually bombarded by chunks of rock and ice in the solar system.
Following this heavy bombardment the early atmosphere of Earth contained. The three H atoms are bonded to the first carbon atom. So your answer would have to be lipids carbohydrates and proteins and nucleic acids.
Found in carbohydrates lipids nucleic acids and proteins. What carbon-based macromolecules are a good source of long-term energy. Depending on the structure these macromolecules can have distinct properties from their monosaccharide building blocks.
Proteins are made of carbon hydrogen. Glucose is a carbohydrate and is the most important simple sugar in human metabolism. Nonpolar covalent bonds polar covalent bonds ionic bonds.
Carbohydrates are found in starch fruits vegetables milk and sugars. Carbon can engage in four covalent bonds. Living things are carbon-based because carbon plays such a prominent role in the chemistry of living things.
Carbon is important to the structure of macromolecules because it has a valence of 4. The four types of macromolecules are carbohydrates lipids proteins and nucleic acids. They are an important source of a healthy diet.
Carbon can bond to itself has 4 valence electrons causing strong covalent bonds to occur between carbon and another element. Sugars contain only carbon oxygen and hydrogen. Examine the statements carefully and mark the correct answer according to the instructions given below.
Carbohydrates sugars and starches Lipids fats and oils Proteins enzymes and antibodies and Nucleic Acids DNA RNA. Complete step by step answer.

0 Comments